BAL Leading the Way Series
A new type of Lyme disease test aimed at early-stage infection detection is hitting doctors’ offices, and we all should thank Lyme patients for making this happen. This test named T-Detect Lyme™, was recently unveiled by Adaptive Biotechnologies, and is an advanced indirect-detection blood test that allows for detection of an acute Lyme infection earlier than antibody response tests.

Our Lyme Disease Biobank (LDB) and Dr. John Aucott’s SLICE Lab at Johns Hopkins University provided the Lyme patient blood samples for Adaptive’s new T-Detect Lyme™ test development. The LDB, a program of Bay Area Lyme, was created in 2014 and began collecting patient samples in 2015 specifically to drive this form of diagnostic innovation. By engaging Lyme patients and providing well-characterized samples to approved researchers and partnering with innovative organizations like Adaptive, the LDB research engine is now delivering long-planned-for results.
“This breakthrough from Adaptive validates the power of patient-driven research. Without the participation of patients who gave blood to our Lyme Disease Biobank, this impactful new test could not have been developed,” commented Linda Giampa, executive director, Bay Area Lyme Foundation. “We wish to thank all the patients who came forward to participate in this important program and to encourage others to give samples.”

The need for Lyme disease patient blood, urine, and tissue samples is ongoing. Giampa predicts that demand will only increase due to many upcoming research projects and partnerships the Foundation is currently pursuing. “We are working with several companies on developing accurate testing for both acute and persistent Lyme infections,” she adds. “Bringing commercial companies into the Lyme disease research ecosphere was an important objective for Bay Area Lyme as diagnostics—and other solutions—will get to market more swiftly.”
Existing tests for the disease look for antibodies to the Lyme bacteria. Because antibodies may take weeks to develop, early testing often yields a false negative. Adaptive’s clinical study utilized their proprietary TCR sequencing and Microsoft’s AI and machine learning to identify a clinical signal for early Lyme disease based on evaluation of the T-cell response to Borrelia burgdorferi, the bacteria that most frequently causes Lyme disease. The T-cell test was shown to be nearly two times more sensitive compared to antibody testing in early Lyme disease, and three times more sensitive in the first few days of symptom onset, supporting observations that T-cell response is detectable before the antibody response.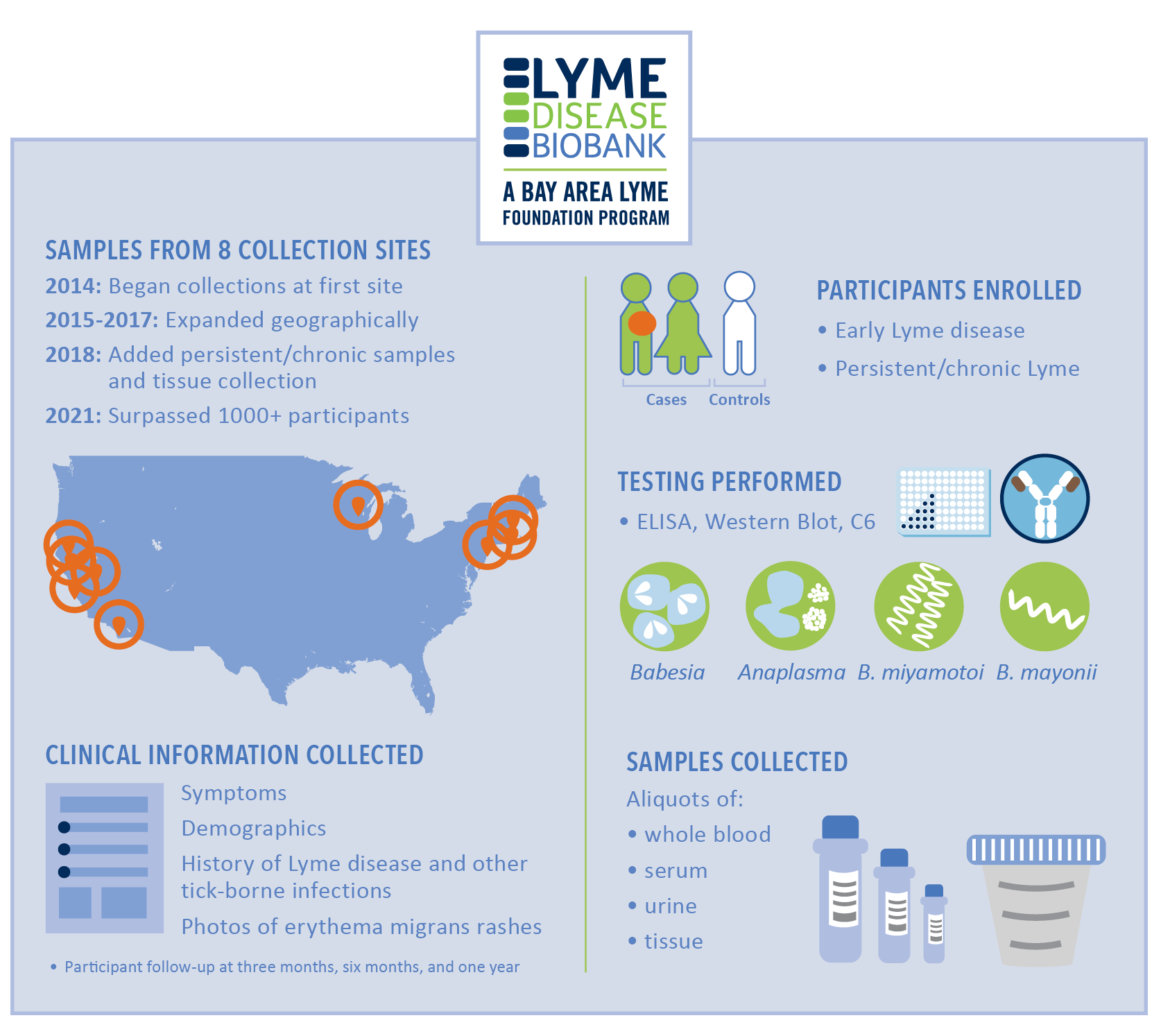
The following is the original press release from Adaptive Biotechnologies:
The T-Detect test detects an immune response by leveraging the body’s unique T-cell response to disease-associated antigens. T-Detect Lyme identifies T cells activated by Borrelia burgdorferi, the bacterium that causes Lyme disease, to help diagnose early Lyme disease.
T-Detect Lyme, administered as a simple blood test, is intended to aid in the diagnosis of early Lyme disease in adult patients demonstrating signs and symptoms.
These symptoms include, but are not limited to, the presence of an erythema migrans (EM) rash, fever, chills, headache, fatigue, muscle and joint aches, and swollen lymph nodes.
Shortening Time to Diagnosis
“The T-Detect franchise leverages the immune system’s natural abilities and the exquisite specificity of T cells to detect disease early,” said Sharon Benzeno, PhD, of Adaptive Biotechnologies, according to the press release issued by the company. “The availability of T-Detect Lyme may help shorten the time to accurate diagnosis for patients with early Lyme disease.”
She continued: “T-Detect Lyme has demonstrated greater sensitivity than serology in early Lyme disease and may aid in the early diagnosis of up to two times more cases that may be missed by traditional serology testing. We aim to further improve the test sensitivity over time using new data sets from additional individuals to be able to help more patients.”
In the US, Lyme disease is the most common tick-borne illness, with approximately half a million newly infected people per year. (1) An early diagnosis of Lyme disease facilitates treatment initiation to stop disease progression. However, today’s standard antibody tests may miss up to 75% of Lyme disease cases in the acute, or early, phase of infection. (2)
More Accurate than Antibody Tests
According to the company, in a clinical validation study among patients with early Lyme disease, the T-cell test was more accurate than leading antibody tests. 3 T-Detect Lyme has a specificity of ~99% and showed more than 1.5 times greater sensitivity than standard two-tiered testing (STTT) in patients who presented with a bullseye rash (54% vs 30%, respectively). (3)
In a separate study, T-Detect Lyme showed three times greater sensitivity than STTT in the first four days after symptoms appeared (44% vs 14%, respectively). (4)
“Lyme disease is a particularly burdensome illness, and the areas where Lyme disease is common are expanding,” said Dr. Shari Rozen, investigator for the ImmuneSense™ Lyme Study.
“The development of antibodies takes time. However, the majority of serologic tests are requested in the early stages of infection, when STTT sensitivity is lower. A T-cell test can improve upon existing options for patients and healthcare providers by measuring a different aspect of the immune response, the T cell, which can arise earlier than antibodies to help identify recent infections.”
T-Detect Lyme is available to patients with a prescription through a qualified healthcare professional as a Clinical Laboratory Improvement Amendments (CLIA) laboratory-developed test (LDT) service.
References
- Centers for Disease Control and Prevention (CDC). Lyme Disease, Data and Surveillance. Available at: https://www.cdc.gov/lyme/datasurveillance/index.html. Accessed June 6, 2022.
- Marques AR. Revisiting the Lyme disease serodiagnostic algorithm: the momentum gathers. J Clin Microbiol. 2018 Jul 26. doi:10.1128/JCM.00749-18.
- Data on file. Adaptive Biotechnologies. 2022.
- Greissl J, et al. Immunosequencing of the T-cell receptor repertoire reveals signatures specific for diagnosis and characterization of early Lyme disease. medRxiv. 2021 Aug 2. doi:10.1101/2021.07.30.21281353.
SOURCE OF PRESS RELEASE: Adaptive Biotechnologies
This Bay Area Lyme Foundation blog is part of our BAL Leading the Way series. Bay Area Lyme provides reliable, fact-based information so that prevention and the importance of early treatment are common knowledge. For more information, including our research and prevention programs, go to www.bayarealyme.org.
We expressly thank the LaureL STEM Fund and the Steven & Alexandra Cohen Foundation for their support of Bay Area Lyme Foundation and our Lyme Disease Biobank program.

