Over half a million Americans continue to struggle for years on end with the pervasive complications of late stage Lyme disease. Lyme disease, which is generally straightforward to treat if caught early, has the potential to become a debilitating long-term illness with symptoms including arthritis, central nervous problems, memory and muscle control issues, and even serious heart conditions. Meanwhile wide variations in symptoms and disease progression and agitated debate about long-term antibiotic use complicate treatment options.
At Bay Area Lyme Foundation, we are excited to support a promising new therapy being developed by Stanford University’s BioMaterials and Advanced Drug Delivery (BioADD) Laboratory. This new treatment leverages commonly used compounds in conjunction to exponentially enhance the impact of the medications. See our past blog post and press release for more information about the discovery.
How Does the Treatment Work?
The combination therapy leverages an antibiotic in conjunction with the “re-purposing” of over-the-counter FDA-approved compounds. In addition to the approved indications, our research has shown that the combination has other useful actions:
1. Blocking Manganese (Mn) from entering the Lyme spirochete and slowing the spirochete’s life-sustaining protein synthesis
2. Protecting the human immune system from antibiotic toxicity by preserving macrophages responsible for removing cellular debris
3. Maximizing the effect of the antibiotic in targeting the harmful bacteria
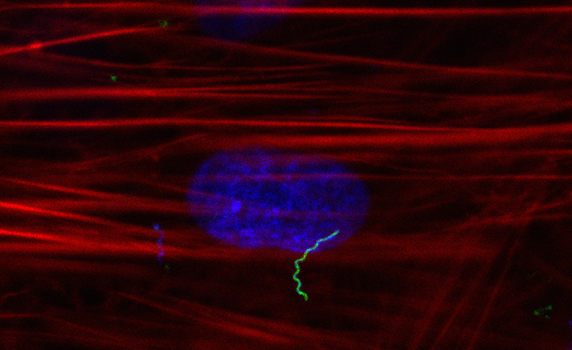
Lyme Clinical Trial Initiative
Finding a cure for Lyme disease or even a solid therapy is a challenging task but we are taking up the charge. Our approach to the problem has two parallel paths:
(1) to identify candidate drugs that are already FDA approved that might be effective for Lyme disease and
(2) to develop a clinical trial protocol that will stand up to the high standards of the FDA and the Lyme community.
In March of 2017, Bay Area Lyme Foundation hosted a Lyme Clinical Trial Summit in Washington, DC where clinical trial and Lyme experts from across the nation gathered to discuss developing a blueprint Lyme clinical trial protocol that could be leveraged for clinicians and researchers seeking to find an effective therapy for Lyme. Topics discussed in the meeting included the challenge of building a rigorously scientific human study in the absence of an accurate diagnostic test, the description of optimal enrollment criteria, the anticipated therapeutic outcomes and how to measure the improvement in ongoing symptoms associated with chronic Lyme infection in a scientifically robust way. The discussion on a consensus clinical trial protocol has progressed with the aim of publishing an open, available blueprint that can be leveraged by all interested parties, worldwide.
In a parallel effort, Bay Area Lyme Foundation is also working to identify candidate drugs from a vast array of compounds that are already FDA approved that may be effective treatments for Lyme alone or in combination with other drugs. The team is evaluating over a dozen prospective drugs suggested by researchers and clinicians and plans to validate the findings with a set of rigorous laboratory and mouse studies to identify most favorable candidates for a clinical trial.
Support
We are grateful to the very generous support of many donors who have already contributed and will help make this treatment an option of the many suffering from late-stage Lyme. Thanks to the many generous Bay Area Lyme supporters, we have already raised more than $850,000 toward the cost of the clinical trial!
Please join us. To make your contribution and bring relief to those suffering from this painful illness, donate today.