BAL Spotlights Series
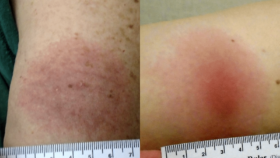
 Anna Schotthoefer, PhD, a project scientist at Marshfield Clinic Research Institute in Wisconsin, discusses the collection and analysis of a specific subset of blood and urine samples for Lyme Disease Biobank—a Bay Area Lyme Foundation program—from patients diagnosed with tick-borne diseases in the state. Marshfield Clinic serves a large population in Wisconsin and Michigan’s Upper Peninsula, which are highly endemic for Lyme disease. Her Bay Area Lyme-funded study of the Marshfield samples focused on visual documentation of rashes associated with Lyme disease and the challenges in accurately diagnosing the disease based on these rashes. The results highlight the difficulties in recognizing early Lyme: only two of 69 patients presented with the classic bullseye rash that doctors learn is the gold standard for diagnosing Lyme from textbooks. Schotthoefer discusses the variety of different rashes that can result from a tick bite, the characterization of the spectrum of rashes, the need for better Lyme diagnostics, and the ongoing efforts to develop new testing methods using the samples collected in LDB. She expresses optimism that in the next five to ten years, there will be significant advancements in Lyme disease detection, diagnosis, and therapeutics—largely thanks to patients who have contributed samples to LDB for ongoing research.
Anna Schotthoefer, PhD, a project scientist at Marshfield Clinic Research Institute in Wisconsin, discusses the collection and analysis of a specific subset of blood and urine samples for Lyme Disease Biobank—a Bay Area Lyme Foundation program—from patients diagnosed with tick-borne diseases in the state. Marshfield Clinic serves a large population in Wisconsin and Michigan’s Upper Peninsula, which are highly endemic for Lyme disease. Her Bay Area Lyme-funded study of the Marshfield samples focused on visual documentation of rashes associated with Lyme disease and the challenges in accurately diagnosing the disease based on these rashes. The results highlight the difficulties in recognizing early Lyme: only two of 69 patients presented with the classic bullseye rash that doctors learn is the gold standard for diagnosing Lyme from textbooks. Schotthoefer discusses the variety of different rashes that can result from a tick bite, the characterization of the spectrum of rashes, the need for better Lyme diagnostics, and the ongoing efforts to develop new testing methods using the samples collected in LDB. She expresses optimism that in the next five to ten years, there will be significant advancements in Lyme disease detection, diagnosis, and therapeutics—largely thanks to patients who have contributed samples to LDB for ongoing research.
“The textbooks doctors read in medical school tell them, ‘Look for a bullseye rash; look for the target-like lesion,’ and it turns out that’s wrong. There is a need to continue educating clinicians and providers that Lyme rashes are a spectrum.”
– Anna Schotthoefer, PhD


 The
The 
 In this blog, part of our 10-year anniversary blog series, we talk with John Aucott, MD, Associate Professor of Medicine at Johns Hopkins University, Director of the Lyme Disease Research Center, about his work and how his investigations are helping us understand persistent/chronic Lyme infections. A long-term collaborator and grant recipient of Bay Area Lyme Foundation, Dr. Aucott reflects on his history with our organization, the ongoing plight of Lyme disease patients, and the slow growth in government funding for investigations into the disease. He talks about the early days of identifying the need for well-characterized samples from Lyme patients and his role in helping launch biobank programs, including his own SLICE Study Biorepository and BAL’s Lyme Disease Biobank.
In this blog, part of our 10-year anniversary blog series, we talk with John Aucott, MD, Associate Professor of Medicine at Johns Hopkins University, Director of the Lyme Disease Research Center, about his work and how his investigations are helping us understand persistent/chronic Lyme infections. A long-term collaborator and grant recipient of Bay Area Lyme Foundation, Dr. Aucott reflects on his history with our organization, the ongoing plight of Lyme disease patients, and the slow growth in government funding for investigations into the disease. He talks about the early days of identifying the need for well-characterized samples from Lyme patients and his role in helping launch biobank programs, including his own SLICE Study Biorepository and BAL’s Lyme Disease Biobank. 


 Dana Parish:
Dana Parish:


