– Bonnie Crater, founder and vice-chair of the Board of Directors, Bay Area Lyme Foundation
What does an anti-alcoholism drug have to do with Lyme disease? Nothing—until a 2016 study funded by Bay Area Lyme Foundation found a link. From around 2014 through 2017, two labs on opposite coasts—one at Johns Hopkins University and one at Stanford—were testing thousands of FDA-approved drugs to identify an existing drug that worked against “persister” forms of Borrelia burgdorferi (Bb), the bacteria that causes Lyme disease(1,2,3,4). Why were they doing this?
Here’s a little background. Borrelia burgdorferi, a spirochete, when cultured in a lab has roughly 3 different forms: a) a culture with predominantly long or corkscrew forms, b) a culture with predominantly round forms and some microcolonies, and c) a culture with predominantly microcolonies (2). Most laboratory studies regarding the effectiveness of antibiotics are conducted in cultures on long forms. In this long form, the spirochete is motile and can divide (although very slowly) and consequently, some antibiotics work much better on the long form. However, after exposure to antibiotics such as doxycycline, the spirochete curls up into a round form and some clump together with other spirochetes to form a few microcolonies. These round-body and microcolony forms are understood to be a defensive posture for the bacteria.
For patients who have the chronic form of Lyme disease, it is hypothesized for the purposes of finding better antibiotics that these patients have persister forms of the bacteria. Dr. Kim Lewis, research professor at Northeastern University published his paper in 2015 and stated matter-of-factly Borrelia burgdorferi, the Causative Agent of Lyme Disease, Forms Drug-Tolerant Persister Cells. And while Dr. Lewis’s work is in the lab (and not on animals or humans), his work has been corroborated by others who found persister Borrelia in mice, dogs, primates, and humans (6-11). In addition, it is widely known that inflammation is a big challenge for chronic Lyme patients which makes them feel poorly overall and have chronic pain symptoms. Consequently, Bay Area Lyme Foundation’s search for new treatments for chronic Lyme conditions included the ability to eliminate all forms of Bb as well as to reduce chronic inflammation associated with Lyme disease.
The exploration of FDA-approved drugs as a Lyme therapeutic is a key strategy for Bay Area Lyme Foundation on our mission “to make Lyme disease easy to diagnose and simple to cure.” Our approach for the last five years has been pretty straightforward: (1) identify borreliacidal drugs in vitro (translation—drugs that kill Bb in a lab), (2) pick the winners and put them into mouse studies and (3) pick the mouse study winners and put them into a clinical trial.
Could we find drugs used for other purposes that kill Borrelia and reduce inflammation? We didn’t know. But Dr. Jayakumar Rajadas, director of Stanford University’s BioADD lab, was on a mission. Dr. Rajadas’s empathy for Lyme patients was notable and he was determined to see if any in a library of FDA-approved drugs would kill long and persister forms of Borrelia and help patients feel better by eliminating the infection and reducing inflammation.
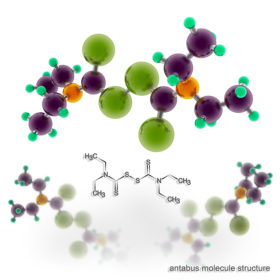
In Dr. Rajadas’s 2016 laboratory study, Identification of New Drug Candidates Against Borrelia burgdorferi using high-throughput screening, post-doctoral researcher Venkata Raveendra Pothineni dedicated himself to the task of running a set of tests on 4,366 FDA-approved compounds. Of the over 4000 drugs screened for activity against Bb, Tetraethylthiuram disulfide was the top candidate. Tetraethylthiuram disulfide, also known as disulfiram and branded Antabuse, is an anti-alcoholism treatment that literally makes a patient have a massive hangover if he or she drinks any alcohol. Disulfiram is known to also have antibiotic properties. (5)
After reading Dr. Rajadas’s 2016 high-throughput screening paper, Dr. Kim Lewis became very curious about disulfiram and ran his own tests with some very interesting results. In October of 2016, Dr. Lewis presented his lab’s work as part of a keynote speech for the Mount Sinai School of Medicine 1st Annual Symposium: Lyme Disease in the Era of Precision Medicine which was sponsored by the Steven & Alexandra Cohen Foundation in New York City. During that speech, Dr. Lewis mentioned the work of Dr. Rajadas’s group showing that disulfiram was a very potent agent against Borrelia burgdorferi persisters. A video recording of Dr. Lewis’s keynote was placed on YouTube. Sometime after that, a patient of Dr. Ken Liegner, who had been following Dr. Lewis’s work, discovered the YouTube video recording of Dr. Lewis’s speech. This individual, an engineer, was doing fairly well on intensive anti-microbial treatment for both Lyme disease and babesiosis but whenever treatment was suspended, he deteriorated with returning symptoms. Like many Lyme patients, the engineer combed the internet for improved methods of treatment that could keep him well.
After finding the YouTube video and bringing it to Dr. Liegner’s attention, the engineer requested Dr. Liegner to start a treatment with disulfiram. The engineer has remained clinically well without need for further treatment for more than two years. Dr. Liegner, who read the publication by Drs. Pothineni and Rajadas which was the basis of Dr. Lewis’s comments, contacted Dr. Rajadas to learn more about his work. After treating several patients with disulfiram, Dr. Liegner published a case series in May 2019 which described his experiences with use of this drug. While not every patient has had as dramatically favorable an outcome as the engineer, Dr. Liegner remains very impressed by the utility of disulfiram and has not seen such dramatic results with the application of any other agent during his 30 years of practice in treating individuals suffering from chronic Lyme disease and chronic babesiosis.
Since then, other physicians have started prescribing disulfiram for patients, including Dr. Richard Horowitz of Hyde Park, New York. In addition, Bay Area Lyme Foundation funded a mouse study with Dr. Monica Embers at Tulane University, Dr. Brian Fallon of Columbia University has initiated a pilot clinical trial, and Dr. Rajadas has continued his work to identify an improved formulation for disulfiram and worked to demonstrate borreliacidal and anti-inflammatory activity in mice. In addition, several Facebook groups have formed for patients are sharing their experience with disulfiram.
While the effectiveness of disulfiram has not yet been evaluated by formal clinical trials and the exact mechanisms by which it exerts its effects on Borrelia is still in the early days of discovery, we know at least some chronic Lyme patients seem to be benefiting from its use. I think we can all be grateful for that. The story of disulfiram is also a story of collaboration across physicians, scientists, foundations, and patients, who are all seeking a cure for Lyme disease. And let the scientists and clinicians continue to collaborate carrying on this important work, while the rest of us cross our fingers that maybe we have gotten lucky and we’ve found a solid treatment that is inexpensive, oral, and relatively safe for chronic Lyme disease.
As a final word, we are not physicians or clinicians at Bay Area Lyme Foundation. If you are a tick-borne disease patient interested in disulfiram, please consult your healthcare provider for further information.
DRUG SCREENINGS REFERENCES:
(1) Feng J, Wang T, Shi W, Zhang S, Sullivan D, Auwaerter PG, Zhang Y. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg Microbes Infect. 2014 Jul;3(7):e49.
(2) Feng J, Auwaerter PG, Zhang Y. Drug combinations against Borrelia burgdorferi persisters in vitro: eradication achieved by using daptomycin, cefoperazone and doxycycline. PLoS One. 2015 Mar 25;10(3):e0117207
(3) Rajadas J. Identification of new drug candidates against Borrelia burgdorferi using high throughput screening, Dovepress 1April 2016: Vol 2016:10 p1307-1322
(4) Pothineni VR, Wagh D, Babar MM, Inayathullah M, Watts RE, Kim KM, Parekh MB, Gurjarpadhye AA, Solow-Cordero D, Tayebi L, Rajadas J. Screening of NCI-DTP library to identify new drug candidates for Borrelia Burgdorferi. J Antibiot (Tokyo). 2017 Mar;70(3):308-312.
(5) Long TE. Repurposing Thiram and Disulfiram as Antibacterial Agents for Multidrug-Resistant Staphylococcus aureus Infections. Antimicrob Agents Chemother. 2017 Aug 24;61(9). pii: e00898-17.
ANIMAL STUDIES REFERENCES:
(6) Barthold SW, Hodzic E, Imai DM, Feng S, Yang X, Luft BJ. Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob Agents Chemother.2010;54(2):643-651.
(7) Hodzic E, Feng S, Holden K, Freet KJ, Barthold SW. Persistence of Borrelia burgdorferi following –
(8) antibiotic treatment in mice. Antimicrob Agents Chemother. 2008;52:1728-1736.
(9) Straubinger RK, Summers BA, Chang YF, Appel MJ. Persistence of Borrelia burdorferi in experimentally infected dogs after antibiotic treatment. J Clin Micobiol. 1997;35(1):111-116.
(10) Embers ME, Hasenkampf NR, Jacobs MB, Tardo AC Doyle-Meyers LA, Philipp MT, Hodzic E. Variable manifestations, diverse seroreactivity and post-treatment persistence in non-human primates exposed to Borrelia burgdorferi by tick feeding. PLoS One. 2017; 12(12):e189071.
(11) Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Eng J Med.1994;330:229-234.
(12) Lawrence C, Lipton RB, Lowy FD, Coyle PK. Seronegative chronic relapsing neuroborreliosis. Eur Neurol. 1995;35(2)113-7.
(13) Chancellor M, McGinnis P, Kilholma P, Hirsch I. Urinary dysfunction in Lyme disease. J of Urol. 1993;149(1):26-30.
DISULFIRAM, CLINICAL APPLICATION FOR LYME DISEASE, REFERENCES
(14) Liegner, Kenneth B. (May 2019). “Disulfiram (Tetraethylthiuram Disulfide) in the Treatment of Lyme Disease and Babesiosis: Report of Experience in Three Cases”. Antibiotics. 8 (2): 72. doi:10.3390/antibiotics8020072
First off, this is a well written article. ❤️ Taking Antabuse was my last resort because I knew deep down if I went to the AA for help, I would try to cut back but would soon slip back to my bad habits. So happy I found ‘Nltrx247’ in Google and got treatment so soon. 👍 I have been taking Antabuse for two weeks and have found that I don’t even want a drink, my life seems much brighter now and full of hope. There is light at the end of the tunnel and it gets brighter every day.
Disulfiram is the meanest shit there is for lyme. You will have nasty lyme herxes. Im almost done killing this bioweapon at 500mg a day for last 8months, 7 months at 250 first, because of violent herxing… It is amazing at killing lyme, it is the cure if you can handle the herxes. You will need bloodwork done every couple months for your liver, and 100mg of b6 for your dopamine imbalance….
I just came across your post. How are you doing Steve? Are you feeling better? I am just going to start disulfiram in a couple of weeks but I am going to start really low at 30mg and work up from there. Any advice??
ALS Formula treatment from Akanni herbal centre , It has made a tremendous difference for me (Visit akanniherbalcentre com). I had improved walking balance
My company provides 100% pure, organic Dihydromyricetin (DHM). The extract is from the Hovenia Dulcis, or ‘Raisin Tree’ as it is commonly known.
One of our customers suffers from chronic lyme disease and she has been prescribed Disulfiram by her doctor.
The medication is currently being proposed as a promising treatment for people suffering from persistent symptoms of Lyme Disease.
Disulfiram, approved by the FDA for the treatment of alcohol abuse, has been shown in the laboratory setting to be effective in the killing of the bacteria that cause Lyme disease.
The side effects can cause severe nausea, vomiting, sweating, and headaches and in rare cases may lead to trouble breathing and irregular heart rhythms.
To use our customer’s words, “This medicine is hard on the liver and causes lots of side effects. Your ‘Raisin Tree’ DHM really helps relieve the nausea and with the detoxing.
I too have late stage Lymes that went too long before being accurately diagnosed.
I’ve had 5 doses of Doxy with relapses shortly after finishing each dose.
I also did 28 days of IV Ceftriaxone. The Infectious Disease clinic wouldn’t consider Disulforam. With no easy way to get it I started going to different doctors. There are 3 ILADS certified doctors in my area. But they do not accept insurance and charge as much as $800.00 per hour.
I called last week and got an appointment with a local physician, which my insurance will cover. I printed out pertinent info about disulforam for Lymes and also quoted to the doctor something I read. “Compassionate Use Request”.
It worked and I’m on my 3rd day of DSL. He prescribed 250mg per day for 30 days to start. He did blood work and will be monitoring my usage every 2 weeks.
At the moment after 3 days I have no adverse effects from DSL and have had no Herx’s as of yet.
My intention is to move up to 500mg per day after 30 days. And stay on it for at least 4 months.
As far as clinical trials, I have been in touch with the one at Columbia.edu. which was suppose to have started last summer. As it stands that has been postponed. The duration is shorter than I’d like and there is also placebo involved. And it requires 16 consultations. 8 in their office (NYC) and 8 over the phone. Due to Wuhan they have pretty much changed the way it will work. Regardless, you will have to be monitored and tested regularly by a doctor during the trial.
It is far more logical to find a local physician that will prescribe it.
I’ll come back here as things progress and pass along my experience.
Good luck everybody.
Contrage Lyme y Babesia en el año 2013. Hoy, tengo 60 años de edad. He desfilado por docenas de médicos de diferentes especialidades. Decenas de miles de dólares en tratamientos y medicinas, por años, y sin cura.
DOXYCICLINA, AMOXIL, CEFALOSPORINAS, QUININA, CEFUROXINA, DAPSONE, ATOVACUONE (MUY COSTOSO) NEEM, TODOS LOS EXTRACTOS DE HIERBAS, DE LA INDIA, DEL ÁFRICA, DE LA AMAZONIA, DEL TIBET Y DE TODOS LOS LUGARES DEL MUNDO, TRATAMIENTOS CON OZONO A LA SANGRE (tres a la semana) y OZONO RECTAL, y el Lyme y la babesia siguen presentes. Los dolores articulares, los dolores de cabeza, la rigidez del cuello y mandíbula, la nube cerebral, en algo han mejorado, pero siguen presentes.
He conseguido ANTABUS de 500 miligramos y lo estoy tomando a diario, con ciertas molestias de sudoración y un poco de somnolencia en las mañanas, con lo cual duermo mejor… Ciento porcento mayor energía y menos dolores articulares, así como una mejor actividad cerebral. Voy 15 dias de ingerirlo y me siento mucho mejor. He aprovechado la cuarentena, para tomarla y veo una gran mejora en mis síntomas. La seguire tomando por tres meses más y publicaré mis resultados… TENGO FE EN ESTE NUEVO TRATAMIENTO…
Has tratado gluthathion?
Si , lo he usado como un desintoxicante. No he sentido un cambio significativo
I’m so happy to share my testimony to the general public today because I had actually given up on life already. When I was diagnosed with deadly sick diseases, I just told God to take my life because the sickness already took over my body. I became a liability to my husband and family. One faithful morning, I was on my bed surfing the web and I saw a testimony on how a man called Dr. Messiah cured deadly virus herpes. If you are positive of COVID-19 stay at home and contact him too, he has a herbal cure. I was a little bit skeptical about it but I decided to give it a try by contacting Dr. Messiah and he assured me on phone I will be well again. In summary of it all, Dr. Messiah cured me. I don’t know how and what he did but his medication worked. Don’t die in silence contact Dr Messiah now via messiahhome@yahoo.com.
As mentioned, Dr. Horowitz integrated DSF to his protocol, but is it replacing Dapsone? Adding your post to our queue on our FB group. https://www.facebook.com/groups/lymecochronicdisease/
This is Michael from Germany again.
About my question regardind my doses plan of Disulfiram anybody is welcome to send me an Email to:
mmems@web.de
Michael, in my experience you are starting with too high a dose of disulfiram. This can cause neuropathy and others side effects. Check with your doctor. My dr has me starting with 62.5mg (compounded into extended release caps) every 3 days x 2 weeks; then every 2 days x 2 weeks; then daily x 2 weeks; then 94 mg daily x 2 weeks; then 125 mg daily x 2 weeks; then I think 184…. and so on until you reach your target dose based on your weight (mine will be 325-350 mg daily for 4 months). Avoid alcohol, fermented things like vinegar, soy sauce, foods high in copper and polyphenols. This is not medical advice, only my experience. Viel Glück!!!
I am Michael, 57 years old from Germany and I suffered on lyme since November 2014 after a tick bite in September 2014.
I tried many antibiotics but nothing helped me.
I started with Disulfiram last week but I am not sure about the doses.
My plan is the following doses:
125 mg every three days for 2 weeks, then
250 mg every three days for 2 weeks, then
125 mg every day for 2 weeks, then
250 mg every day for 2 – 3 month.
Is that doses OK or it to fast / much ??
Please let me know if that is OK or not.
Thank you!!
Hi Carmen did you ever find a source?
Drop me an email-
nick@juicepossum.com
I am a NY resident and have had Lyme for about 18 years, but did not get a conclusive diagnosis for many, many years due to MD ignorance. At the outset, I suspected Lyme and asked for doxycycline treatment (in 2002). The first treatment (10 days, I think) made me feel better, so I asked for and was Rx’d a second round, which made me feel about 95% back to normal. I asked for, but was refused, a third round. All my symptoms came gradually back.
I then began years of pain and misery, going from doctor to doctor, trying to discover the cause of my many seemingly unrelated symptoms (all of them). Insurance did not pay for the many tests and treatments I tried. I had to stop working. I tried many different antibiotics and herbal and vitamin regimens, none of which had any effect. I pretty much gave up and just tried to keep going, despite worsening symptoms. My IgG bands were always at 8 out of 10. I was caring for my two elderly parents and had little time for my own healthcare.
In 2017, I once again managed time to look for medical help. I went on a 30-day course of IV Rocephin, which did nothing to alleviate my symptoms, but inflicted me with 18 gallbladder attacks (finally confirmed by ultrasound tests), which my infinitely-uninformed MD assured me were “just Herx reactions”. I DID get a reduction in IgG (IgM? always forget which is which – the 10-band test) bands from 8 to 5 during a course of Rife treatments in Spring/Summer 2018, but I didn’t really FEEL any better and the treatments were $$$ so I quit.
In March 2019, I heard about the Columbia Antabuse trials, contacted them and tried to get on the trials myself. They did not accept me as they had very strict parameters into which I apparently did not fall (the test group was very small). I then went to my GP and alerted him to this trial. I asked if he knew anything about Antabuse, which he did, and he agreed to prescribe it to me in the dose used in the Columbia trial, which its available online at clinical trials.gov. Not knowing anything about “starting low and going slow,” I took 500 mg per day divided into 2 doses for 4 weeks with the result that I began to feel better and better. Fatigue and joint pain vanished, energy returned, mental clarity returned and how! Depression gone. I went out and got a JOB. (Too much, too soon, and not enuf money, so I did give it up.) Got bloodwork after the first 4 weeks, which showed IgG bands gone down from 8 to 5. Began to feel positively euphoric – like my old self.
Then someone told me that one side effect of Antabuse is a “high” which can then turn around to a precipitous “crash.” Fearing that I was actually getting into such a pattern, I cut back on the dose to 250/d. I also began to feel the “drowsy” and “tired” effects. TIRED, but nicely so, like after a hard day of hiking, say. Just wanted to sleep a lot – not that awful dragged out exhaustion of Lyme. My second “4 weeks” of Antabuse actually took me more than 8 weeks, as I was taking only half the dosage and occasionally just took a couple days off when the sleepiness just got overwhelming. I also began to experience mild headache and nausea. Finally finished all the prescribed Antabuse, waited two weeks, got bloodwork done again and got the news that my bands were down to 3, from 5. (Officially “cured.”)
My MD and I decided that I should continue with the Antabuse for another 4 week round in order to try to get the bloodwork down to NO bands. I am now in the second week of the third round. Tiredness and nausea are again an issue – and I also find my balance problems exacerbated (this is my worst ongoing problem). I, therefore, am experimenting now with a “pulsed” dousing regimen: 250 mg/d for seven days followed by a break of however long it takes til I feel better (less tired, no nausea) – typically 3-4 days – then repeat seven days at 250 mg/d, etc. I will continue this till pills are used up, then do bloodwork again. This makes sense to me, because I figure that the Lyme microbes are probably killed off as the drug floods my bloodstream, so I feel toxic – then the Lyme retreats and I feel better. The Lyme will then begin to reemerge as the drug disappears and so I hit it again with the next “pulse.” We shall see.
Meanwhile, I want to thank the wonderful MDs who have brought us this treatment and — I hope and pray — CURE. Especially, I want to thank my MD who prescribed me this drug without any fuss. He’s a good man and I recommend him verbally to everyone, but will not name him here in case it could get him in some kind of trouble for prescribing “off-label.” Ask your MDs to give you this treatment – just watch out for the sleepiness side effect – I fell asleep for a few moments on the NYS Thruway at 65 mph and woke up as I was running off into the shoulder – I do not recommend any lengthy road trips while on this drug. Good luck, everyone!!!
I am a NY resident and would like to get the name of the doctor verbally. I am trying to persuade my doctor to prescribe but I am not having much luck. Thank you!
I will be starting Antabuse treatment in 2 weeks. Interestingly, it was my general practitioner MD who told me about it yesterday during a routine appointment. I had already heard about it but did not realize it could be prescribed so easily. I run Lyme Expressions and Fellowship (LEAF) FB page and will post updates for as long as I’m on the treatment. I am extremely sensitive to Lyme treatment (antibiotic) and quit antibiotic treatment after 11 years the herxheimer reactions were simply unbearable. So we’ll see how well I can handle a herx on Antabuse.
Hi S Cranswick, thank you so much for your precious input, I really appreciated it! At moment I’m taking DSF and experiencing pain in the gallbladder as well. Is it possible to PM you?
Interestingly the common inky cap mushroom does the same thing as Disulfiram. Will it work for lyme as well?
I had difficulty swallowing and fatigue. I was given medications which helped but only for a short burst of time, then I decided to try alternative measures and began on ALS Formula treatment from Akanni herbal centre. It has made a tremendous difference for me (Visit http://www.akanniherbalcentre.com). I had improved walking balance, increased appetite, muscle strength, improved eyesight, and others.
First off, this is a well written article. ❤️ Taking Antabuse was my last resort because I knew deep down if I went to the AA for help, I would try to cut back but would soon slip back to my bad habits. So happy I found ‘Nltrx247’ in Google and got treatment so soon. 👍 I have been taking Antabuse for two weeks and have found that I don’t even want a drink, my life seems much brighter now and full of hope. There is light at the end of the tunnel and it gets brighter every day.
Christine Jonckheer wrote in Dutch:
Wonderful !!!! Are the side effects so bad? I react very fiercely to many treatments. Do you have to take it for life? Can any doctor prescribe it and does it cost a lot? (financial well after 20 years of illness and all kinds of treatments) Thankful if you want to answer me.
My answer:
I’m in the middle of my treatment. It is very important to take Disulfiram LOW AND SLOW and if you do this you can control most of the BAD side effects. You do not need to take it for life. I hope 6 months will be enough. I visited Dr Wolfgang Klemann in Pforzheim, Germany to get the prescription. He also gave me laboratory orders to be able to test liver, kidney and blood every 3-4 weeks. The cost is approximately 25 euro/month.
Hi Karen, are you still taking Disulfiram? I have a prescription from the same doctor as you and started with it around 9 weeks ago. How are you doing now after nearly six months? Kind Regards, David
I just finished my treatment and I’m following up with a year of low-dose naltrexone. I started in November and it’s now July 2. The last 8 weeks or so I’ve had bad neuropathy but it’s slowly fading and after the last ten-15 years of hell I’ve been through, the side effects are worth it. I also had a herx that sent me to the hospital, so make sure you have plenty of support. I feel incredible. I’m 37 and after years of feeling 87, I now feel 22. I pray for everyone to heal from this horrible, misunderstood illness. Now that they found a cure, people are finally believing our stories. It’s took too long but at least we’re here now. Be well and safe.
Hi Karen,
I saw you mentioning the name of dr klemann. I have my first appointment in his practice in 4 weeks. I feel so terrible i think i will die before i get to see him. I started taking disulfiram 4 days ago but i dont feel any improvement yet. How soon did you start to feel better with disulfiram? Thanks.
This is the first possible treatment I have found for my dear suffering Husband. He has plenty of symptoms and neuropathy in a wheelchair from being an active strong man to this…:-( Galaxy labs found Bartonella and Babesia. Our LLMD will not give us this drug at this time. Please how can we get this drug. Nothing has been working and it has cost us thousands. Our LLMD is very cautious about it and now has him on ABART and Mino low doses building up incrementally. But it has been 2.5 years already and 30 drs. We need help. Please let us know what you can do. I am Carmen, his Wife. thank you!!
I have found some benefit from disulfiram and I’m reading many success stories on Facebook. I had to stop due to side effects but will likely restart at a lower dose. We need follow up studies to determine the best course of treatment and work to improve drug delivery and stability so a lower dose can be used with fewer side effects. Please fund more of this important work.
May I ask what your side effects were? I have been on 125mg for the past two weeks and just increased to 250mg yesterday. In two more weeks, I will increase to 500mg. I have been very tired and sleepy since beginning Disulfiram, also spacey and lightheaded. It’s always hard to know if its a side effect from the drug or an increase in the chronic Lyme symptoms. Thank you and I wish you the best!
Thank you Bay Area Lyme for writing such a clear piece about this discovery and how it has become popular. I hope it is shared everywhere and Jayakumar Rajadas’ lab receives FULL credit and additional funding to continue their work for us patients. I have been on it for 6 months now including a 6-week break and feel soooo GOOD! I’ve treated for 7 years to no avail with drug combos, ozone, herbals, homeopathy, LDI, etc. and still got Lyme and coinfections back!
This drug is not easy at times but NOTHING patients should be afraid of using a low and slow approach. A lot of the fear that has circulated in a support group has been from people NOT on the drug and from those who tried to go too high and too fast. There is good patient support in a healing environment at Disulfiram for Lyme Group. Anyone can PM me for questions or join us in our group to learn about the drug and how it might help you or a family member. My Dr just called it the cure for Lyme yesterday. COMPLETELY FLOORED ME! He’s not the only one saying that! I hope BAL will support this lab with as much funding as they can.
Prachtig!!!!Zijn de bijwerkingen zo erg? Ik reageer nl heel heftig op veel behandelingen. Moet je het levenslang nemen? Kan elke dokter het voorschrijven en kost het veel? (financiele put na 20 jaar ziek en behandelingen allerlei) Dankbaar als u me wil antwoorden.
I would like to join your group!
Hi, Good info. Suffering for over 12 years. 3 months of IVs, the works. Felt toxic from antibio. How did you know coinfections were back?. Put in for three months of disulfiram. Wish me luck. Tired of being crash test dummy.
Thank you so much for the information, Kristina. I have Lyme Carditis and pretty much every other physical and neurological symptom with increasing severity over the past 3 years. I would love to join your group and discover what others are doing with Disulfiram. I’m particularly interested in what I may expect, recommended dosage, what groups have seen the most positive impact, and finding some fellow sufferers I can relate with. Thank you!! Brandon Capes
Please let me know how to get into the Disulfiram for Lyme Group. My husband’s doctors just prescribed this for him today. Thank you
How do we get in your Facebook group? Would like to join.
For those looking for the facebook group, see here: https://www.facebook.com/disulfiramforlyme
Hi Can you help me? My husband just started it — he takes 250 mg broken into 4 so takes 1/4 every other day and is herxing badly. Is he starting too high? He’s on it 2 weeks and wants to stop. Thank you.
Kristina Bauer I would like to ask you some questions!
I live in Canton, GA and I need a dr who prescribed this. I have a Lyme a very long time.
Thank you for posting this story about the ground-breaking work done by Dr Rajadas’ and Venkata Pothineni‘s amazing team for the treatment of chronic and acute Lyme disease. Their discoveries of medications that are actually effective in treating patients who have been sick for years are nothing short of revolutionary. This small group of researchers has accomplished more in a few years than has been done in the past 35+ years. These scientists are, truly, the largely unsung heroes who deserve our admiration and support to continue their efforts to give Lyme patients back their lives.
I’m so proud of Rajadas’s team. Keep it up.
I am very excited to read your article! This is printed and will be taken to two of my doctors along with other important articles on Disulfiram and pioneer Drs and researchers. The FB Disulfiram Support Groups are being followed daily and I watch carefully the posts.
I believe these infections are the root cause of many serious debilitating “diseases,” including suicide to escape. It is a living hell to suffer from all the miserable life-sucking symptoms. Please know we are so grateful for the devoted pioneers and true humanitarian researchers.
Bless you all and God speed your works. Waiting for a cure🙏🏼
Will Bay Area Lyme be finding more of Dr Rajadas research? I would like to donate.
Bay Area Lyme is excited about the promise of this research to offer real therapeutic relief for Lyme patients and also for the collaboration and innovation which it represents. We hope to continue to support efforts like these. You can find more information about how to donate here.
I am a rare 29-year late-stage Lyme, with Babesia duncani client with cardiac interested pathogens and I have many colonies. I am a nutritionist or I would not be here. I am interested in this drug. I would like to be contacted.
We will pass on your contact information to the researchers and you will be added to the database of potential participants for future clinical studies. You may be interested in the following information about participating in clinical studies. Best wishes.
They will not contact you. Call all the ILADS doctors in your state and ask if they offer this. ILADS has a list per state on their website.