By Bay Area Lyme Foundation FOR IMMEDIATE RELEASE
Journal of Clinical Microbiology Studies Demonstrate Two Investigational Diagnostics Outperform Current Tests in Detecting Early Lyme Disease
Studies utilize Bay Area Lyme Foundation’s Lyme Disease Biobank samples to point to the promise of single-tier diagnostics to potentially transform early detection
PORTOLA VALLEY, Calif., October 9, 2025 — Bay Area Lyme Foundation, the leading nonprofit funder of Lyme disease research in the US, today announced results from two independent studies published in Journal of Clinical Microbiology, conducted by researchers at Tufts University School of Medicine, and Kephera Diagnostics, respectively, demonstrating the potential of novel investigational single-tier Lyme disease tests to improve accuracy in the earliest stages of infection. Each study uses well-characterized samples from Bay Area Lyme Foundation’s Lyme Disease Biobank and demonstrated unprecedented accuracy, far exceeding the current CDC-recommended Lyme disease two-tier test, which can miss up to 70% of early-stage cases as well as later-stage cases.
“The CDC’s standard two-tier Lyme diagnostic misses the majority of early cases, delaying treatment and increasing the risk of developing persistent, debilitating symptoms for patients. The two novel single-tier assays—while not yet FDA-cleared for clinical use—point to a future where Lyme disease can be diagnosed quickly, accurately, and with a single test,” Liz Horn, PhD, MBI, a coauthor on both studies, and Principal Investigator of Lyme Disease Biobank, a Bay Area Lyme Foundation program that provides much-needed samples to approved researchers working to better understand tick-borne diseases and develop improved diagnostic tests and therapeutics. “These single tier tests, like InBios Lyme Detect™ and Kephera’s Hybrid Lyme ELISA could mark a turning point for Lyme diagnostics, giving physicians and patients more accurate tools that are urgently needed.”
The first study, evaluating the InBios Lyme Detect™ Multiplex ELISA, was conducted by Pete Gwynne, PhD, a 2022 Bay Area Lyme Emerging Leader Award (ELA) winner, and colleagues at Tufts University School of Medicine. Using samples from the Lyme Disease Biobank, this new diagnostic correctly identified all two-tier positive samples evaluated in the study, while also detecting 21 of 79 clinically diagnosed patients who were missed by following the current CDC guidance for testing using FDA-cleared standard two-tier tests (STTT) and had erythema migrans (EM) skin lesions. Importantly, the InBios test maintained >99% specificity, with only one false positive across more than 200 control and lookalike disease samples and was shown to be highly reproducible.




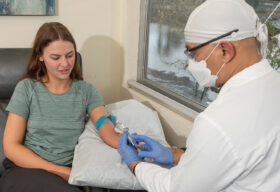








 Lyme Disease Biobank established a key partnership with the nonprofit
Lyme Disease Biobank established a key partnership with the nonprofit  “Although it is an emotional and difficult idea for anyone to plan to donate parts of their body to science after they have died, we believe that this decision is an important way for Lyme patients to change the course of Lyme disease research. Having access to tissues from the brain, heart, joints, and central nervous system of Lyme patients allows researchers to prove unequivocally that Lyme is present in tissue and contributes to patient suffering,” explains
“Although it is an emotional and difficult idea for anyone to plan to donate parts of their body to science after they have died, we believe that this decision is an important way for Lyme patients to change the course of Lyme disease research. Having access to tissues from the brain, heart, joints, and central nervous system of Lyme patients allows researchers to prove unequivocally that Lyme is present in tissue and contributes to patient suffering,” explains