– Wendy Adams, Research Grant Director, Bay Area Lyme Foundation
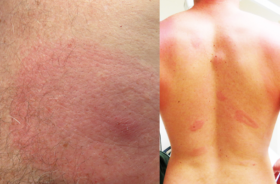
Erythema migrans (EM) is the hallmark sign of infection with B. burgdorferi. An EM is defined as an expanding annular (round) lesion or rash of at least 10cm (2.5in). Most rashes occur 3–30 days after infection, however there are case reports that show EMs can appear sooner than three days post infection.
The term “bullseye” rash is often used synonymously with EM. But an EM is not required to have central clearing or a target appearance. The rash can take many forms, and may have a raised bump in the middle, can be itchy or warm, and can have a bluish cast like a bruise. It can be round or even oval. Only 20% of Lyme disease with an EM have the bullseye presentation. That means that only 1 in 6 total Lyme cases will have a rash with a target appearance.
The rash also may not be present at all. While the Centers for Disease Control and Prevention report that 70-80% of patients may exhibit the erythema migrans, this number can vary by study. For example, a 2010 study showed that in the state of Maine only 43% of Lyme patients exhibited this rash when infected with Lyme.