BAL 10-year Anniversary Series
10 Years of Collaboration Bringing Hope: How Dr. John Aucott’s Relationship with Bay Area Lyme Helped Get Groundbreaking Biobanks Launched to Fuel the Research Engine of Lyme Disease Investigations
 In this blog, part of our 10-year anniversary blog series, we talk with John Aucott, MD, Associate Professor of Medicine at Johns Hopkins University, Director of the Lyme Disease Research Center, about his work and how his investigations are helping us understand persistent/chronic Lyme infections. A long-term collaborator and grant recipient of Bay Area Lyme Foundation, Dr. Aucott reflects on his history with our organization, the ongoing plight of Lyme disease patients, and the slow growth in government funding for investigations into the disease. He talks about the early days of identifying the need for well-characterized samples from Lyme patients and his role in helping launch biobank programs, including his own SLICE Study Biorepository and BAL’s Lyme Disease Biobank.
In this blog, part of our 10-year anniversary blog series, we talk with John Aucott, MD, Associate Professor of Medicine at Johns Hopkins University, Director of the Lyme Disease Research Center, about his work and how his investigations are helping us understand persistent/chronic Lyme infections. A long-term collaborator and grant recipient of Bay Area Lyme Foundation, Dr. Aucott reflects on his history with our organization, the ongoing plight of Lyme disease patients, and the slow growth in government funding for investigations into the disease. He talks about the early days of identifying the need for well-characterized samples from Lyme patients and his role in helping launch biobank programs, including his own SLICE Study Biorepository and BAL’s Lyme Disease Biobank.
Bay Area Lyme: I want to take you right back to the very beginning of your relationship with Bay Area Lyme (BAL), the founding of your SLICE study and our Lyme Disease Biobank (LDB) and talk about everything that was happening 10 years ago. People talk about the “norming, storming and forming” stages of organizations, and there was an awful lot of activity going on 10 years ago in the world of Lyme disease. And so, please share your thoughts on what was happening around that time, your part in it, and how you came into the picture with BAL and our biobank.

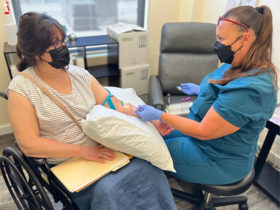
John Aucott: My first memory is that I flew out to California and my agenda at that time was getting people interested in research. There was very little funding for Lyme disease research, and to some extent there still isn’t a great amount of funding for Lyme disease, especially the kind of research I do, which is clinical translational research. I’m an MD, so my research involves bridging basic science to human beings to patients. So, to be very candid about it, I was interested in getting BAL interested in my work. I pitched what we were doing. We had already set up the Johns Hopkins SLICE study, the Study of Lyme disease Immunology and Clinical Events. And at that point it was one of only two large scale biobanks collecting for Lyme disease. The other one is Dr. Gary Wormser’s, who still has a biobank in Valhalla, New York.
So, I was pitching the idea of a Lyme disease biobank to BAL, and this was a whole new concept. A biobank is a hard concept to sell initially because people don’t understand that setting up a biobank is like being Levi Strauss: It’s selling the Levis and the picks, and the shovels—not selling them the actual gold in your pocket. And it’s a hard pitch because people don’t understand that somebody has to supply the foundational work so that other people can mine for the gold. But BAL caught on to the idea that the biobank that we had at Hopkins was a crucial resource that would enable collaboration with other researchers to advance the scientific understanding of disease mechanisms and potentially identify and validate biomarkers for improving diagnostics and treatments.
Bay Area Lyme bought into the importance of this idea. The first thing that happened was you asked me to be on the BAL Scientific Advisory Board soon thereafter. BAL expressed support for the SLICE study’s biobank at Hopkins and you actually gave us one of our first grants to help support our biobank work. So, that was the first thing that happened. We applied for a grant, and we got a grant from BAL to help us because we had not yet gotten federal funding for it. So, that was one of the first grants to support it, and now in 2023 we are finally receiving our first NIH funding.