Thank you for your interest in learning more about Lyme Disease Biobank®. We hope you will join us to help solve the challenges of Lyme and tick-borne diseases. Simply click on the buttons below to find out more about the different ways we are working with you—whether you’re a patient, a healthcare provider, a researcher, or you have a loved one who has Lyme—we invite you to be part of this initiative.
What is a Biobank?
A biobank, also known as a biorepository, stores human biological samples like blood and tissue for use in future research. Lyme Disease Biobank was created because medical researchers were unable to access the human blood, urine, and tissue samples they needed for their research. This lack of samples made it extremely difficult to conduct research on new diagnostics and treatments for Lyme and other tick-borne diseases. Read more about the history of the biobank below.
About Lyme Disease Biobank
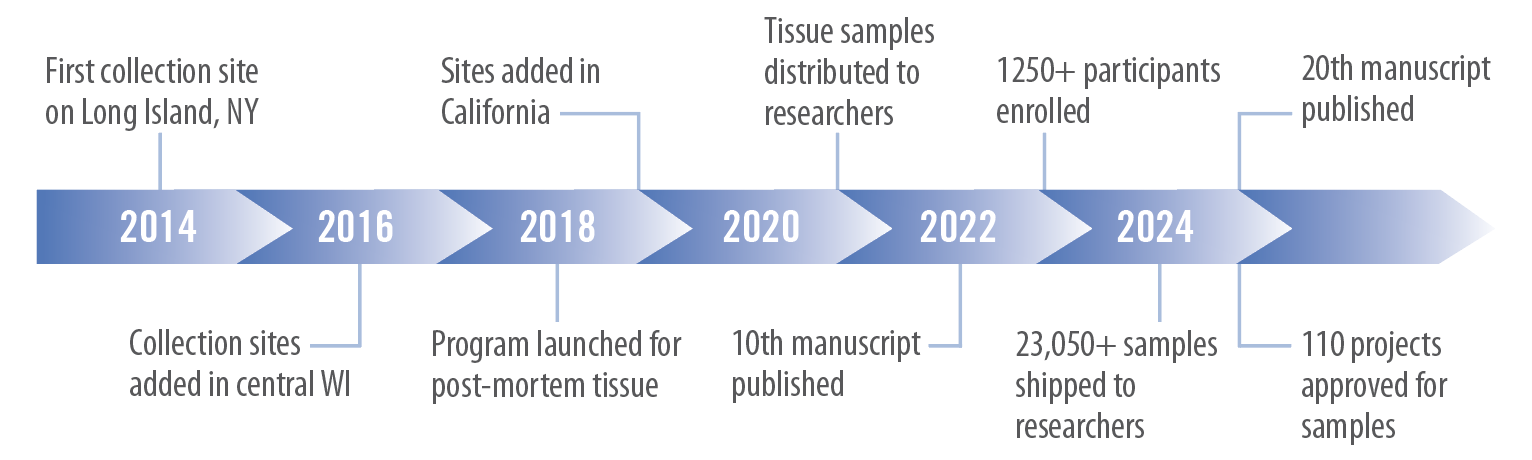
Lyme Disease Biobank was launched with a Fund-A-Need campaign at Bay Area Lyme Foundation’s annual LymeAid® benefit in May of 2014. That same year, collection began with a pilot study on Long Island, NY. Since then, our Biobank has enrolled more than 1,250 participants from eight collection sites located on the East Coast, Upper Midwest, and in California.
This research program is designed to provide blood, urine, and human tissue samples to investigators working on better diagnostics and new treatments for Lyme and other tick-borne diseases. We are collecting samples from people with early Lyme, as well as from people with chronic/persistent Lyme. By collecting tissue samples, the Biobank helps researchers study how pathogens (bacteria, parasites, and viruses) invade human tissues. All samples include detailed medical and health histories and testing results.
At this time, our blood and urine samples are enabling more than 110 research projects in academia and industry.
If you have questions about this program, please email Liz Horn, PhD, MBI, Principal Investigator, Lyme Disease Biobank.
Lyme Disease Biobank is a supporting organization of the Bay Area Lyme Foundation and is headquartered in Portland, Oregon. Since 2016, our Biobank has been supported by the LaureL STEM Fund, Bay Area Lyme capital campaigns, and a generous donation from the Steven & Alexandra Cohen Foundation.
Current Lyme Disease Biobank Results
- 110 projects approved for samples
- 20th manuscript published
- 23,050+ samples shipped to researchers (through 2024)
- 1250+ participants enrolled
Timeline of Lyme Disease Biobank Development
For a more detailed timeline click here.
People
Biobank Board
Wendy Adams, Research Grant Director, Bay Area Lyme Foundation
Bonnie Crater, Founder and Co-Chair of the Board of Directors, Bay Area Lyme Foundation
Linda Giampa, Executive Director, Bay Area Lyme Foundation
Principal Investigator
LDB’s success is made possible through the collaborative spirit of our partners and volunteers. We are incredibly grateful to the following people and organizations whose contributions are essential for making the research engine hum.
Biobank Participants: Biobank participants voluntarily provide blood, urine, and tissue samples to the program. Our participants include people with Lyme and other tick-borne diseases as well as people without these diseases.
Clinical Sites: LDB clinical collection sites enroll participants across the country, and each site’s Principal Investigator (PI) provides guidance to the project.
Peer Reviewers: More than 40 experts volunteer their time to review applications from sample users. These reviewers provide recommendations on which research projects should receive samples.
Sample Users: Approved researchers in academia and industry use LDB samples to test new diagnostics and improve our understanding of Lyme and other tick-borne diseases.
Working Group Participants: Working group participants volunteered their time to provide expertise and advice on the development of the Lyme Disease Biobank and the Biobank Tissue Bank Program.
Partner Organizations
MyLymeData: MyLymeData helps generate awareness about the Tissue Collection Program and provides additional information about the tissue samples to sample users.
NDRI: (National Disease Research Interchange) NDRI manages the logistics of the Tissue Collection Program, working directly with tissue donors, surgical staff, and others to coordinate sample collection.
Precision for Medicine: Precision for Medicine processes and stores LDB samples and sends samples to researchers.
Research Foundation for Mental Hygiene: This organization provides sample processing specific to the Tissue Collection Program as well as neuropathology expertise.
Testing Partners: Our Biobank testing partners test Biobank samples to help further characterize them. Our testing partners include Mayo Clinic, Stony Brook University, and New York Medical College. Samples are tested for Lyme using serology assays and for tick-borne infections using PCR.
We would like to recognize and thank the Steven & Alexandra Cohen Foundation, the LaureL STEM Fund, an anonymous donor, and the 2014 & 2018 LymeAid® Fund-a-Need supporters who have contributed significantly to our Lyme Disease Biobank.
We are grateful to the participants who volunteered and donated samples.
Explanations of Frequently Used Terms
What do we mean by acute/early Lyme?
Acute, or “early” Lyme disease symptoms may begin hours, a few days, or even weeks after a bite from an infected tick. This stage is called “acute” because this is the first stage of Lyme disease. Acute Lyme symptoms can overlap with those of other infections, including viruses like influenza and SARS-CoV-2 and other tick-borne infections and may include headache, fever and/or chills, fatigue, muscle and body aches, and joint pain. Lyme disease is often but not always accompanied by an expanding skin rash called an erythema migrans (EM) that develops at the site of the tick bite and sometimes may have a central clearing like a bull’s eye or target. As the bacteria move around the body (disseminate) multiple Lyme rashes and other symptoms (cardiac, neurologic, arthritic) may appear. To read more about acute Lyme symptoms click here.
What do we mean by chronic/persistent Lyme?
When Lyme is diagnosed early and treated with an appropriate course of antibiotics, most people get better. However, for some people, Lyme disease is not diagnosed until later, when the infection has disseminated throughout the body. For others who were diagnosed with early disease and treated, the initial course of antibiotics may not be enough to clear the infection. In both scenarios, people can have ongoing/persistent symptoms of Lyme disease. These chronic/persistent symptoms can include fatigue, sleep impairment, joint pain, muscle pain, other pain, cognitive impairment (including memory issues, burning/stabbing sensations, brain fog), neuropathy (weakness or numbness typically in the hands and feet), or other symptoms. To read more about chronic/persistent Lyme symptoms, click here.
What do we mean by clinical information?
Each sample includes clinical information that tells us more about the person’s experience with Lyme disease and their health history. This clinical information is important for researchers using the samples. Samples are collected at a specific point in time, just like a picture is a snapshot of one point in time. However, life is more like a movie or continuum of pictures, and clinical information helps provide context about the person who donated the sample. This includes if the person recalls a tick bite and their experiences around tick exposure, if they have a rash and what it looks like (a picture is also taken), any symptoms they are experiencing, any antibiotics that are prescribed (or already taken?), if they have a history of Lyme or other tick-borne diseases, information about their general health, and any medications they are currently taking.
What do we mean by “pathogens”?
Pathogens are bacteria, viruses or parasites that can cause disease in humans. When we refer to pathogens, we are talking about all of the different microbes that ticks can pass to humans through bites that cause disease. For example, Lyme disease, the most common tick-borne disease, is usually caused by the bacteria Borrelia burgdorferi, while babesiosis is usually caused by the parasite Babesia microti. Ticks can be infected by multiple pathogens at the same time.
What do we mean by “tissue”?
Tissue is a group of cells with similar structure that act together to perform a specific function. The human body is made up of cells, tissues, and organs. LDB is collecting tissue samples to look for evidence of infection and evidence of inflammation. There are two ways to collect tissue: one is during a planned surgery, and another is after someone has died (post-mortem). For example, tissue such as cartilage can be collected from a knee replacement, while tissues from the brain and heart are usually collected after someone has died. You may find more information in NDRI’s FAQ here.
What do we mean by “well-characterized” samples and why is this important?
Biobank samples are described as “well-characterized” because they include clinical information about each donor’s experience with Lyme disease and health history, as well as results from laboratory tests at the time the sample was collected. These tests include tests to measure Lyme antibodies, and tests to detect other tick-borne pathogens the participant might have also been exposed to. Without testing the samples at the time of collection, research results on that sample are harder to interpret. The more information LDB collects on a sample, the more useful it is to approved researchers as they interpret their findings and results.
Is there a cost to participate in Lyme Disease Biobank?
There is no cost to families or patients when donating blood, urine, or tissue.
You may also find comprehensive information about Lyme and other tick-borne diseases on our website here.
If you have questions about this program, please email Liz Horn, PhD, MBI, Principal Investigator, Lyme Disease Biobank.
We would like to recognize and thank the Steven & Alexandra Cohen Foundation, the LaureL STEM Fund, an anonymous donor, and the 2014 & 2018 LymeAid® Fund-a-Need supporters who have contributed significantly to our Lyme Disease Biobank.
We are grateful to the participants who volunteered and donated samples.
